Barcodes at the supermarket allow fast and easy product identification, often including such valuable information as location, quantity, and tracking.
Now, a rapid gene labeling or characterization scheme for bacteria-infecting viruses known as bacteriophages, or phages, has been developed for similar purposes by researchers at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab).
Based on the Nobel-Prize winning CRISPR gene-editing technology, the scientists’ method allowed them to understand what parts of a phage genome are essential to its function. Once nonessential regions are identified, barcodes in these regions would allow investigators and clinicians to quickly identify and track different phages in diverse settings. Brought to scale, the method stands to unlock powerful biotechnology applications relevant to agriculture, the environment, human health, and more.
For example, with the new barcoding method, investigators would be able to track phages while they are being used to manipulate the microbiome around plant roots to enhance plant growth in drought conditions or without the use of fertilizers. They could also track barcoded phages being used to treat serious conditions like antibiotic-resistant infections.
“The impact of this work is immense,” said Vivek Mutalik, a staff scientist in Berkeley Lab’s Biosciences Area and a corresponding author of a PLOS Biology paper describing the method. “This report is the first studying gene essentiality at this scale in bacterial viruses.”
Mutalik said he and his co-authors demonstrate, on a genome-wide scale, that they can identify phage genes that are essential (or not) to infecting bacteria. It’s what scientists call “gene essentiality.” What’s more, the knowledge gained for one type of phage can be extended to others.
“By knocking down expression of each gene at genome-wide scale, we can assess which genes in these two phages are important and which ones are dispensable [nonessential] for the infection cycle.”
– Vivek Mutalik
“We use CRISPR interference (CRISPRi) technology to dial down almost every gene in two model phages for mapping the gene essentiality landscape,” Mutalik said. “By knocking down expression of each gene at genome-wide scale, we can assess which genes in these two phages are important and which ones are dispensable [nonessential] for the infection cycle.”
CRISPRi has been used in mammalian and bacterial systems, Mutalik said. He added that the work opens a door for similar studies across different phages, and for building genotype to phenotype mapping, thus providing guidance for future innovators. “We also uncovered how phage genes are highly connected and that knocking down one gene impacts the expression of [another] downstream gene, a phenomenon known as ‘polarity,’” he said.
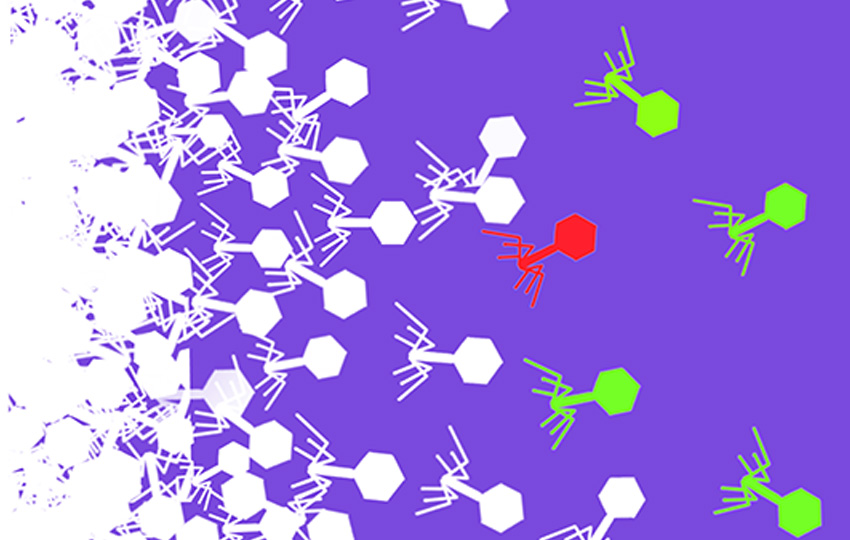
While it may seem counterintuitive, Mutalik said, the goal of this recent work was finding nonessential genes in phage genomes. And that’s also where barcoding comes in.
“The question was, once we identify a nonessential gene, can we replace that with some identifier – a unique tag,” said Mutalik. “It’s like at a grocery store where you have barcodes on every item. Imagine somebody drops stuff all over the grocery store. How does the shopkeeper know where to go to put everything back? They can use the barcode to know which aisle, where, and so on. The barcodes help organize and standardize the entire workflow and help in how we handle different items in a collection. The same thing can happen with phage cocktails.”
“Let’s say I make a phage cocktail – a mixture of phages with unique barcodes on them – to use in an application,” Mutalik continued. “But then we want to follow how phages are performing. To do this, we can simply find and track the barcodes. This process will help us find which phages in that phage cocktail are working and which ones are not. Imagine after five days we find that a particular phage mixture treatment is not working and needs some changes made. Then we can change the phage cocktail maybe by removing one or two phages and adding some more interesting ones that are better [in an application]. Using barcoded phages will enable this entire process to be much more streamlined.”
Phages are the most abundant biological entities on Earth. They represent one of the largest pools of genetic diversity in nature, albeit with mostly unexplored gene-function information. The new CRISPRi characterization method, Mutalik said, might help unleash a range of useful biotech applications like pathogen control in agriculture, environmental remediation, food and dairy production, gene therapy, vaccine development or ridding medical devices of dangerous biofilms that often build up and threaten device functioning as well as patient health.
The gene-function information for bacteriophages is like “an ocean of knowledge we don’t know how to tap into,” Mutalik said. “On the one hand there is the untapped gene information, and then on the other hand there is the application space, for example, antibiotic resistance, where we need some urgent solutions. There are so many potential phage applications out there.”
Despite their potential, however, phages have remained poorly characterized, Mutalik said. In part, that’s because gene characterization methods, to date, have been labor-intensive and specific to certain phage types as to not be useful given the immense universe of phages.
“If we want to study genomic architecture of hundreds of phages important in diverse applications, we need to improve these [characterization] methods further,” Mutalik said.
Recognizing this, DOE’s Biopreparedness Research Virtual Environment (BRaVE) initiative, which supports national biopreparedness and response capabilities, has just funded the “Phage Foundry.” Under Mutalik’s leadership, the Phage Foundry brings together multidisciplinary and multi-institutional expertise to develop a foundational platform that integrates many aspects of phage research to enable rapid development of targeted phage-based therapeutics against antimicrobial-resistant pathogens.
“The future is bright for phage research,” Mutalik said.
This research was funded by DOE’s Office of Science Biological and Environmental Research and DOE’s National Virtual Biotechnology Laboratory.
###
Lawrence Berkeley National Laboratory (Berkeley Lab) is committed to delivering solutions for humankind through research in clean energy, a healthy planet, and discovery science. Founded in 1931 on the belief that the biggest problems are best addressed by teams, Berkeley Lab and its scientists have been recognized with 16 Nobel Prizes. Researchers from around the world rely on the Lab’s world-class scientific facilities for their own pioneering research. Berkeley Lab is a multiprogram national laboratory managed by the University of California for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.